Multiple Choice
The stability of 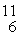 C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 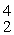
He: 4.002603 u 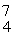
Be: 7.016928 u 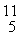
B: 11.009305 u 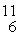
C: 11.011433 u 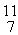
N: 11.026742 u
The 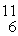
C nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Consider two different isotopes of the same
Q9: In massive stars,three helium nuclei fuse together,forming
Q52: Find the reaction energy (Q value) of
Q53: An air sample is contaminated with <sup>15</sup>O,
Q54: Fermium-253 has a half-life of 3.00 d.
Q55: A hospital patient has been given some
Q56: The carbon in your body was formed
Q59: Radium-226 decays into radon-222 plus an alpha
Q61: Today, the uranium found on Earth contains
Q62: An excited <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="An excited