Multiple Choice
Find the reaction energy (Q value) of the reaction 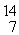 N +
N + 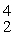
He → 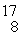
O + 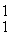
H, given the following masses: 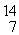
N: 14.003074 u 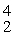
He: 4.002603 u 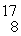
O : 16.999131 u 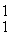
H: 1.007825 u
(1 u = 931.5 MeV/c2)
A) -1.191 MeV
B) -2.030 MeV
C) -3.241 MeV
D) -6.724 MeV
E) -9.055 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Heavier stable nuclei tend to have<br>A) half
Q43: Which of the following statements about the
Q47: A stationary plutonium-239 nucleus decays into a
Q49: Living matter has 1.3 × 10<sup>-10</sup> %
Q53: An air sample is contaminated with <sup>15</sup>O,
Q54: Fermium-253 has a half-life of 3.00 d.
Q55: A hospital patient has been given some
Q56: The carbon in your body was formed
Q57: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="The stability
Q82: The radioactivity due to carbon-14 measured in