Multiple Choice
Two deuterium nuclei, 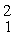 H, fuse to produce a helium nucleus,
H, fuse to produce a helium nucleus, 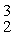
H, and a neutron. A neutral deuterium atom has a mass of 2.014102 u; a neutral helium atom has a mass of 3.016030 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
A) 3.27 MeV
B) 3.57 MeV
C) 4.00 MeV
D) 4.50 MeV
E) 5.68 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q8: The maximum permissible workday dose for occupational
Q20: Going from medium mass nuclei to heavy
Q65: A radioactive sample has a half-life of
Q71: What would be the expected radius of
Q73: The unstable isotope <sup>234</sup>Th decays by β
Q74: The neutral deuterium atom, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="The
Q75: A sphere made of a radioactive isotope
Q77: How much energy is released in the
Q82: Consider the fusion reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Consider
Q89: How does the mass of the products