Multiple Choice
Consider the reaction of 2-chloro-2-methylpentane with sodium iodide. 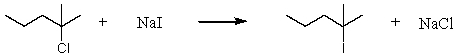 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
A) No effect
B) It would double the rate.
C) It would triple the rate.
D) It would quadruple the rate.
E) It would increase the rate five times.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Draw the potential energy diagram that represents
Q64: The substitution mechanism whose rate depends primarily
Q98: The product(s)for the following reaction would mainly
Q99: Which S<sub>N</sub>2 reaction will occur most
Q100: What would be the major product(s)of the
Q101: What would be the major product(s)of the
Q104: By analyzing the starting material and the
Q105: Which would be formed in the following
Q107: Which is the weakest nucleophile in polar
Q108: Which of the following would be most