Multiple Choice
Which of the following would be most reactive in an SN2 reaction?
A) 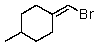
B) 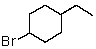
C) 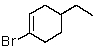
D) 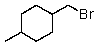
E) 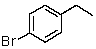
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: The substitution mechanism whose rate depends primarily
Q103: Consider the reaction of 2-chloro-2-methylpentane with sodium
Q104: By analyzing the starting material and the
Q105: Which would be formed in the following
Q107: Which is the weakest nucleophile in polar
Q109: What would be the major product(s)of the
Q110: Select the potential energy diagram that represents
Q111: What major product(s)are likely to be obtained
Q112: Which alkyl halide,when treated with sodium ethoxide
Q113: By analyzing the starting material and the