Multiple Choice
Use the following diagram to answer the next problem. 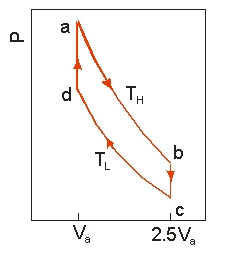 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
A) 22.9 J
B) 30.5 J
C) 7.62 J
D) 8.31 J
E) 0.917 J
Correct Answer:

Verified
Correct Answer:
Verified
Q45: A heat engine with an output of
Q46: A steam power plant with an efficiency
Q47: A steam power plant with an efficiency
Q48: A refrigerator with a coefficient of performance
Q49: The efficiency of a Carnot engine operating
Q51: Two refrigerators,one with a COP of 4.0
Q52: Use the following diagram to answer the
Q53: A heat engine absorbs 64 kcal of
Q54: A refrigerator has a coefficient of performance
Q55: A substance undergoes a series of reversible