Multiple Choice
Use the following diagram to answer the next problem. 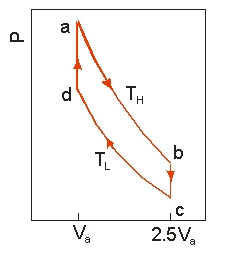 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second,the power output is
A) 381 W
B) 45.8 W
C) 1145 W
D) 415 W
E) 1525 W
Correct Answer:

Verified
Correct Answer:
Verified
Q47: A steam power plant with an efficiency
Q48: A refrigerator with a coefficient of performance
Q49: The efficiency of a Carnot engine operating
Q50: Use the following diagram to answer the
Q51: Two refrigerators,one with a COP of 4.0
Q53: A heat engine absorbs 64 kcal of
Q54: A refrigerator has a coefficient of performance
Q55: A substance undergoes a series of reversible
Q56: A heat engine operating between the temperatures
Q57: A heat pump needs to supply