Multiple Choice
A hydrogen atom is in a state with principal quantum number n = 3.A possible value for its orbital angular momentum is
A) zero
B) 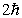
C) 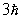
D) 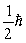
E) 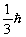
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: A gas-discharge tube filled with hydrogen gas
Q82: The possible orbital quantum numbers of an
Q83: An electron undergoes a transition from n
Q84: A K<sub> <span class="ql-formula" data-value="\beta"><span class="katex"><span
Q85: What is the ratio of the radius
Q87: The binding energy of hydrogen is
Q88: If the potential energy of an electron
Q89: The electron in a hydrogen atom
Q90: For the hydrogen atom in the
Q91: The principle quantum number of an electron