Multiple Choice
For the hydrogen atom in the ground state,the radial probability density is 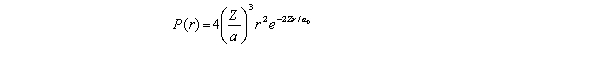 The probability of finding the electron in range r = 0.08a0 at r = a0 is approximately
The probability of finding the electron in range r = 0.08a0 at r = a0 is approximately
A) 3.24%
B) 4.33%
C) 5.87%
D) 6.25%
E) 7.43%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: What is the ratio of the radius
Q86: A hydrogen atom is in a state
Q87: The binding energy of hydrogen is
Q88: If the potential energy of an electron
Q89: The electron in a hydrogen atom
Q91: The principle quantum number of an electron
Q92: Mosely showed that the wavelength of
Q93: The first Bohr radius,r<sub>0</sub>,is 0.0529 nm and
Q94: J.J.Thomson's model of an atom<br>A)had electrons embedded
Q95: What is the energy difference between the