Multiple Choice
Use the following figure for the next problem. 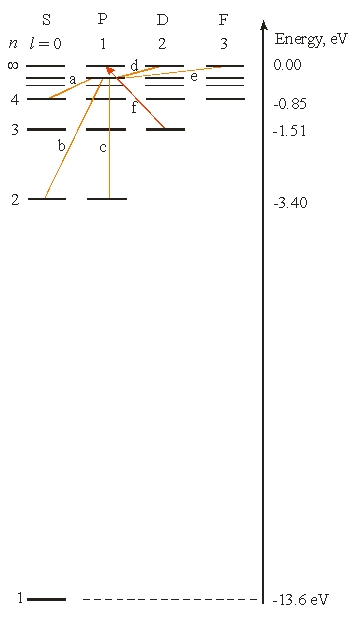
-What is the longest wavelength needed for an electron to make the transition labeled (f) ?
A) 151 nm
B) 365 nm
C) 422 nm
D) 821 nm
E) 1459 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: Use the following figure for the next
Q104: The equation derived by Bohr for
Q105: The number of oxygen's eight electrons that
Q106: What is the difference in wavelength between
Q107: A photon of wavelength 80 nm is
Q108: Z for the element whose electronic configuration
Q109: The energy of the nth level in
Q110: The orbital angular momentum L is related
Q111: The constant in the Rydberg-Ritz formula is
Q113: The wavelength of the photon emitted