Multiple Choice
Use the following to answer the question: 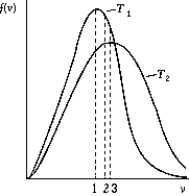
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
A) The curve labeled T2 represents the distribution for the higher temperature molecules.
B) The point labeled "1" corresponds to the rms speed of the molecules whose temperature is T1.
C) The point labeled "2" corresponds to the maximum speed of the molecules whose temperature is T1.
D) The point labeled "3" corresponds to the average speed of the molecules whose temperature is T1.
E) None of these is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q50: If the absolute temperature of a gas
Q51: A device used to measure the
Q52: A temperature of 14ºF is equivalent to<br>A)-10ºC<br>B)7.77ºC<br>C)25.5ºC<br>D)26.7ºC<br>E)47.7ºC
Q53: Use the following to answer the question:
Q54: On the basis of the kinetic theory
Q56: Boltzmann's constant,k,has a value of 1.381
Q57: The rms speed of oxygen molecules is
Q58: A temperature difference of 9ºF is the
Q59: Normal human body temperature is 98.6ºF.What is
Q60: A constant-volume gas thermometer reads 6.66 kPa