Multiple Choice
Which of the following structure is an acceptable bond line formula for CH3CHClCHCH2? 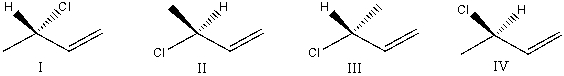
A) I
B) I and IV
C) II and III
D) I,II,and III
E) All of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Draw all the isomers of C<sub>4</sub>H<sub>9</sub>Br,using bond-line
Q17: Select the hybridized atomic orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q18: Which of the following represent a pair
Q19: Consider the following: CH<sub>3</sub>CH<sub>2</sub>CH=CHCH<sub>2</sub>CH<sub>3 </sub><sub> </sub><sub> </sub>CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CH<sub>2</sub><br>I
Q20: When the 1s orbitals of two hydrogen
Q22: The bond angle for the C-P-C
Q23: What would be the spatial arrangement of
Q24: Contributing resonance structures cannot have contributors whose
Q25: Define an orbital.
Q26: In quantum mechanics a node (nodal