Multiple Choice
Select the hybridized atomic orbital. 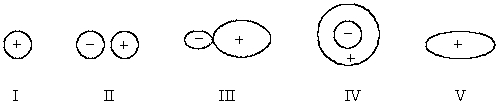
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q12: CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub> and CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH are examples of what
Q13: Identify the atomic orbital the lone pair
Q14: Which of the following species is/are not
Q15: If a tetrahedral carbon atom were to
Q16: Draw all the isomers of C<sub>4</sub>H<sub>9</sub>Br,using bond-line
Q18: Which of the following represent a pair
Q19: Consider the following: CH<sub>3</sub>CH<sub>2</sub>CH=CHCH<sub>2</sub>CH<sub>3 </sub><sub> </sub><sub> </sub>CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CH<sub>2</sub><br>I
Q20: When the 1s orbitals of two hydrogen
Q21: Which of the following structure is an
Q22: The bond angle for the C-P-C