Multiple Choice
Which of the following pairs are NOT resonance structures?
A) 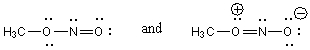
B) 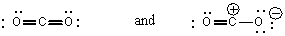
C) 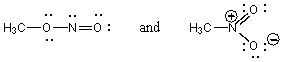
D) Each of these pairs represents resonance structures.
E) None of these pairs represents resonance structures.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: What is the hybridization of the N
Q56: The major resonance contributor to the resonance
Q57: Which of the following species contributes more
Q58: Which of the following structures represent compounds
Q59: Which of the following is the Lewis
Q61: Identify the atomic orbitals in the C-N
Q62: Identify the atomic orbital the lone pair
Q63: The bond angle for the C-N-O
Q64: In which of the following would you
Q65: The following electron configuration represents: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"