Multiple Choice
The following electron configuration represents: 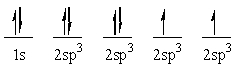
A) the ground state of nitrogen
B) the ground state of oxygen
C) the sp3 hybridized state of carbon
D) the excited state of oxygen
E) None of these choices correctly identifies the given electron configuration.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: Which of the following pairs are NOT
Q61: Identify the atomic orbitals in the C-N
Q62: Identify the atomic orbital the lone pair
Q63: The bond angle for the C-N-O
Q64: In which of the following would you
Q66: Which of the following species is a
Q67: Which molecule has a non-linear shape
Q68: Which type of bonding is present in
Q69: Which of the following species contributes more
Q70: Identify the atomic orbital the lone pair