Multiple Choice
Which of the following species is a resonance form of the species in the box? 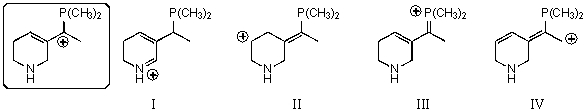
A) I
B) II
C) III
D) IV
E) None of these choices are correct resonance forms.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: Identify the atomic orbitals in the C-N
Q62: Identify the atomic orbital the lone pair
Q63: The bond angle for the C-N-O
Q64: In which of the following would you
Q65: The following electron configuration represents: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q67: Which molecule has a non-linear shape
Q68: Which type of bonding is present in
Q69: Which of the following species contributes more
Q70: Identify the atomic orbital the lone pair
Q71: Which is NOT a correct Lewis structure?<br>A)