Multiple Choice
Which of the following is/are not a resonance form(s) of the species in the box? 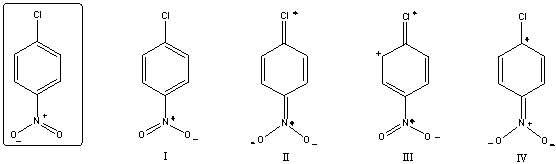
A) I
B) II
C) III
D) IV
E) More than two of these choices are incorrect resonance forms.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: In which molecule(s)can the molecular geometry be
Q34: What is the geometry around the N
Q35: Nonbonding lone electron pairs exist in the
Q36: Which of the following would have a
Q37: The bond angle for the C-C-N
Q39: Expansion of the valence shell to accommodate
Q40: The formal charge on sulfur in sulfuric
Q41: The following electron configuration represents: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q42: Which of the following species contributes more
Q43: Which principle(s)or rule(s)must be used to determine