Multiple Choice
The following electron configuration represents: 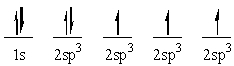
A) the ground state of boron
B) the sp3 hybridized state of carbon
C) the sp3 hybridized state of nitrogen
D) the ground state of carbon
E) an excited state of carbon
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Which of the following would have a
Q37: The bond angle for the C-C-N
Q38: Which of the following is/are not a
Q39: Expansion of the valence shell to accommodate
Q40: The formal charge on sulfur in sulfuric
Q42: Which of the following species contributes more
Q43: Which principle(s)or rule(s)must be used to determine
Q44: All organic compounds have their origins from
Q45: What is the approximate hybridization state of
Q46: What geometry does the methyl cation,CH<sub>3</sub><sup>+</sup>,have?<br>A)octahedral<br>B)tetrahedral<br>C)trigonal planar<br>D)linear<br>E)trigonal