Multiple Choice
Which of the following species is/are not a resonance form(s) of the species in the box? 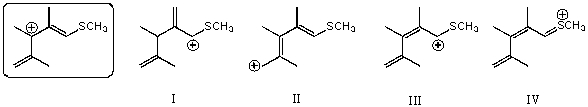
A) I
B) II
C) III
D) IV
E) More than two of these choices are incorrect resonance forms.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q130: Which compound is not a constitutional isomer
Q131: What is the hybridization of the O
Q132: Organic compounds were originally defined as compounds
Q133: When two waves with equal amplitude and
Q134: How many 2p atomic orbitals from boron
Q136: The bond angle for the C-C-O
Q137: Identify the atomic orbital the lone pair
Q138: Identify the hybridized orbitals involved in the
Q139: Which is the shortest of the carbon-carbon
Q140: How many total sigma bonds are present