Multiple Choice
Identify the hybridized orbitals involved in the C-2-C-3 sigma bond (indicated by an arrow) in the following molecule: 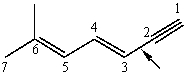
A) sp2,sp2
B) sp2,sp
C) sp2,sp3
D) sp3,sp2
E) sp,sp2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q133: When two waves with equal amplitude and
Q134: How many 2p atomic orbitals from boron
Q135: Which of the following species is/are not
Q136: The bond angle for the C-C-O
Q137: Identify the atomic orbital the lone pair
Q139: Which is the shortest of the carbon-carbon
Q140: How many total sigma bonds are present
Q141: Select the most electronegative element from the
Q142: Which structure(s)contain(s)an oxygen that bears a formal
Q143: According to molecular orbital theory,which molecule could