Multiple Choice
Which of the following species is/are not a resonance form(s) of the species in the box? 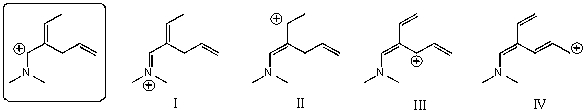
A) I and II
B) II and III
C) III and IV
D) I and IV
E) II and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Which of these substances contain both covalent
Q10: Which of the following best describes the
Q11: Which compound is not a constitutional isomer
Q12: CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub> and CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH are examples of what
Q13: Identify the atomic orbital the lone pair
Q15: If a tetrahedral carbon atom were to
Q16: Draw all the isomers of C<sub>4</sub>H<sub>9</sub>Br,using bond-line
Q17: Select the hybridized atomic orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q18: Which of the following represent a pair
Q19: Consider the following: CH<sub>3</sub>CH<sub>2</sub>CH=CHCH<sub>2</sub>CH<sub>3 </sub><sub> </sub><sub> </sub>CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CH<sub>2</sub><br>I