Multiple Choice
Rank the following hydrogen atoms from highest to lowest in acidity in the structure shown below. 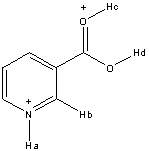
A) Ha > Hb > Hc > Hd
B) Ha > Hc > Hd > Hb
C) Hc > Hd > Ha > Hb
D) Hd > Ha > Hc > Hb
E) Hc > Ha > Hd > Hb
Correct Answer:

Verified
Correct Answer:
Verified
Q83: What does the reaction between the following
Q84: What is/are the product(s)of the following acid-base
Q85: Carboxylic acids have conjugate bases that are
Q86: Rank the bold-faced hydrogens for the following
Q87: For the simple hydrides,MH<sub>n</sub>,pK<sub>a</sub> values decrease in
Q89: Adding sodium amide (NaNH<sub>2</sub>)to 1-butyne (CH<sub>3</sub>CH<sub>2</sub>C≡CH)would produce:<br>A)CH<sub>3</sub>CH<sub>2</sub>C≡CNa
Q90: Which might be used as a protic
Q91: Protons or other electron-deficient centers that seek
Q92: For the following acid-base reaction,which statement
Q93: Which base would not effectively deprotonate phenol