Multiple Choice
Which might be used as a protic solvent?
A) 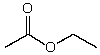
B) 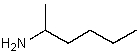
C) 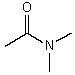
D) 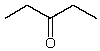
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Carboxylic acids have conjugate bases that are
Q86: Rank the bold-faced hydrogens for the following
Q87: For the simple hydrides,MH<sub>n</sub>,pK<sub>a</sub> values decrease in
Q88: Rank the following hydrogen atoms from highest
Q89: Adding sodium amide (NaNH<sub>2</sub>)to 1-butyne (CH<sub>3</sub>CH<sub>2</sub>C≡CH)would produce:<br>A)CH<sub>3</sub>CH<sub>2</sub>C≡CNa
Q91: Protons or other electron-deficient centers that seek
Q92: For the following acid-base reaction,which statement
Q93: Which base would not effectively deprotonate phenol
Q94: What is/are the product(s)of the following acid-base
Q95: It is impossible to have pK<sub>a</sub> values