Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 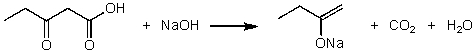
A) The reaction is an exothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is positive.
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Why cannot one determine the relative acid
Q33: For the following acid-base reaction,which statement
Q34: Rank the bold-faced hydrogens for the following
Q35: The isomer of alanine shown below is
Q36: For the following acid/base reaction which
Q38: What are the two fundamental types of
Q39: Which base would not effectively deprotonate acetylene?<br>A)i-PrMgBr<br>B)KH<br>C)CH<sub>3</sub>OCH<sub>2</sub>Li<br>D)(i-Pr)<sub>2</sub>NLi<br>E)t-BuOK
Q40: The basic species are arranged in decreasing
Q41: Adding sodium hydride,NaH,to water produces:<br>A)H<sub>2</sub> and NaOH(aq)<br>B)H<sup>-</sup>(aq)+
Q42: Which combination of reagents is effective in