Essay
The isomer of alanine shown below is one of the 20 naturally occurring amino acids that are used to make proteins.Amino acids like alanine exist at neutral acidity (pH = 7)in the following form: 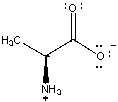 What would be the structure of alanine if HCl(aq)was added to lower the pH = 1? What would be the structure of alanine if NaOH(aq)was added until the pH = 12?
What would be the structure of alanine if HCl(aq)was added to lower the pH = 1? What would be the structure of alanine if NaOH(aq)was added until the pH = 12?
Correct Answer:

Verified
Correct Answer:
Verified
Q30: Which base would most effectively deprotonate benzoic
Q31: Which reaction of these potential acids and
Q32: Why cannot one determine the relative acid
Q33: For the following acid-base reaction,which statement
Q34: Rank the bold-faced hydrogens for the following
Q36: For the following acid/base reaction which
Q37: For the following acid/base reaction which
Q38: What are the two fundamental types of
Q39: Which base would not effectively deprotonate acetylene?<br>A)i-PrMgBr<br>B)KH<br>C)CH<sub>3</sub>OCH<sub>2</sub>Li<br>D)(i-Pr)<sub>2</sub>NLi<br>E)t-BuOK
Q40: The basic species are arranged in decreasing