Multiple Choice
The reaction, 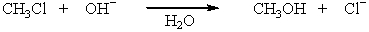 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
A) -73.8 kJ mol-1
B) -76.8 kJ mol-1
C) -59.0 kJ mol-1
D) +91.6 kJ mol-1
E) -91.6 kJ mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: Increasing the temperature of a chemical reaction
Q82: Which of the following alkyl bromide isomers
Q83: Increasing the temperature of a reaction will
Q84: The difference in the bond energies
Q85: Select the potential energy diagram that represents
Q86: An increase in the kinetic energy of
Q87: Which is the weakest nucleophile in polar
Q88: In a highly exergonic S<sub>N</sub>2 reaction we
Q89: Which of the following is not true
Q90: A true statement about the transition state(s)of