Multiple Choice
Consider the reaction of 2-chloro-2-methylpentane with sodium iodide. 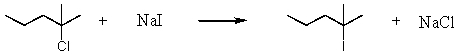 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would quadruple the rate.
E) It would increase the rate five times.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Reaction of (R)-2-chloro-4-methylhexane with excess NaI in
Q12: Typically alkyl chlorides are slow in S<sub>N</sub>2
Q13: Considering the relative solvation of reactants
Q14: An endergonic S<sub>N</sub>2 reaction will have
Q15: Draw the potential energy diagram that represents
Q17: Which of the following would be most
Q18: The rate equation for a nucleophilic substitution
Q19: Which S<sub>N</sub>2 reaction would take place most
Q20: Which ion is the strongest nucleophile in
Q21: Racemic mixtures form in S<sub>N</sub>1 reactions when