Multiple Choice
Which of the following would be most reactive in an SN2 reaction?
A) 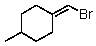
B) 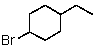
C) 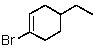
D) 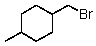
E) 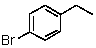
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Typically alkyl chlorides are slow in S<sub>N</sub>2
Q13: Considering the relative solvation of reactants
Q14: An endergonic S<sub>N</sub>2 reaction will have
Q15: Draw the potential energy diagram that represents
Q16: Consider the reaction of 2-chloro-2-methylpentane with sodium
Q18: The rate equation for a nucleophilic substitution
Q19: Which S<sub>N</sub>2 reaction would take place most
Q20: Which ion is the strongest nucleophile in
Q21: Racemic mixtures form in S<sub>N</sub>1 reactions when
Q22: The rate equation for a nucleophilic substitution