Multiple Choice
A compound with the molecular formula C6H15N gave the following 1H NMR spectrum: triplet, 0 0.90
Quartet, 2.4
There were no other signals.The most likely structure for the compound is:
A) 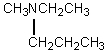
B) 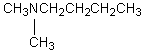
C) CH3CH2CH2CH2CH2CH2NH2
D) 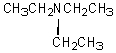
E) 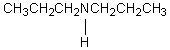
Correct Answer:

Verified
Correct Answer:
Verified
Q100: Determine the most likely structure of
Q101: Consider the expected splitting of the C2
Q102: Briefly explain how you might distinguish between
Q103: For the following compound how many different
Q104: Consider the expected splitting of signal "b"
Q106: Which form of electromagnetic radiation possesses the
Q107: Consider the expected splitting of signal "c"
Q108: An acceptable solvent to use for a
Q109: What is the structure of the compound
Q110: For the compound adamantine,how many different signals