Multiple Choice
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12O which shows no characteristic stretches in the IR between 3600-3300 cm-1,but does around 1600 cm-1? Relative integration is shown. 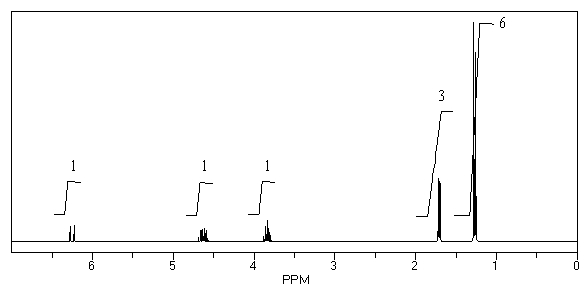
A) 
B) 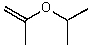
C) 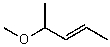
D) 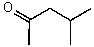
E) 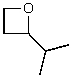
Correct Answer:

Verified
Correct Answer:
Verified
Q104: Consider the expected splitting of signal "b"
Q105: A compound with the molecular formula
Q106: Which form of electromagnetic radiation possesses the
Q107: Consider the expected splitting of signal "c"
Q108: An acceptable solvent to use for a
Q110: For the compound adamantine,how many different signals
Q111: In the structure shown,H<sub>b</sub> and H<sub>c</sub> are
Q112: For the following compound how many different
Q113: An unknown compound,F,has the formula C<sub>3</sub>H<sub>6</sub>O<sub>2</sub>.Elucidate the
Q114: How many <sup>13</sup>C signals would 1,2-dimethylbenzene give?