Multiple Choice
The specific heat of copper is 0.0920 cal/g °C,and the specific heat of silver is 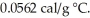 If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
A) The copper will reach a higher temperature.
B) The silver will reach a higher temperature.
C) The two samples will reach the same temperature.
D) The copper will reach a temperature lower than 25 °C.
E) The silver will soften.
Correct Answer:

Verified
Correct Answer:
Verified
Q100: The phrase "ability to do work" is
Q101: A slice of pizza contains 28 g
Q102: A 12-g sample of cooking oil,which is
Q103: Which of the following is a chemical
Q104: A serving of fish contains 50 g
Q105: The specific heat of water (in J/g
Q106: A car driving on the freeway has
Q107: How many calories are required to increase
Q108: If the heat of vaporization for water
Q109: A liquid has its own volume and