Multiple Choice
In a buffer system of HF and its salt,NaF,________.
A) the HF neutralizes added acid
B) the HF neutralizes added base
C) the HF is not necessary
D) the 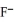 neutralizes added
neutralizes added 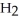 O
O
E) the 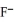 neutralizes added base
neutralizes added base
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The correct formula for sulfuric acid is
Q3: A solution with [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="A
Q4: The conjugate base of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The
Q5: A solution with a pH greater than
Q6: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The name
Q7: For <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="For ,the
Q8: The name given to an aqueous solution
Q9: KOH is a strong base.
Q10: Alkalosis is the blood condition in which
Q11: The name of NaOH is sodium hydroxide.