Multiple Choice
Which of the following molecular geometries would most likely give rise to a nonpolar compound?
A) 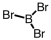
B) 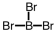
C) 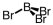
D) 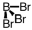
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following would you expect
Q16: NH<sub>3</sub> (ammonia)exhibits what type of structure?<br>A)triangle<br>B)pyramid with
Q23: Which of the following electronic changes will
Q30: Which of the following molecules would be
Q44: Some elements can display more than one
Q66: The correct name for BaCO<sub>3</sub> is _
Q71: Which of the following would be a
Q79: The correct formula of an ionic compound
Q91: The rigid three-dimensional arrangement assumed by the
Q93: Which type of interparticle forces leads to