Multiple Choice
The correct formula of an ionic compound containing Fe2+ and ClO 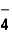 is _____ .
is _____ .
A) FeClO4
B) Fe2(ClO4) 3
C) Fe(ClO4) 2
D) Fe3(ClO4) 2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following has the highest
Q23: Which of the following electronic changes will
Q33: The shape of a BF<sub>3</sub> molecule is
Q40: HCl and HBr can exhibit hydrogen bonding
Q45: Atoms or ions are considered isoelectronic if<br>A)they
Q66: The correct name for BaCO<sub>3</sub> is _
Q71: Which of the following would be a
Q74: Which of the following molecular geometries would
Q81: Which of the following is the correct
Q93: Which type of interparticle forces leads to