Multiple Choice
The number n of gas particles in a leak-tight container is approximately described by the equation 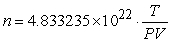 , where T is the temperature of the gas in kelvins (K) , P is the pressure of the gas in atmospheres (atm) , and V is the volume of the container in liters (L) . For a particular sample, an experimenter finds T = 296.6 0.01 K, P = 1.05 0.01 atm, and V = 1.302 0.002 L. Use a linear approximation to estimate the range of the computed value of n.
, where T is the temperature of the gas in kelvins (K) , P is the pressure of the gas in atmospheres (atm) , and V is the volume of the container in liters (L) . For a particular sample, an experimenter finds T = 296.6 0.01 K, P = 1.05 0.01 atm, and V = 1.302 0.002 L. Use a linear approximation to estimate the range of the computed value of n.
A) 0 to 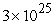
B) 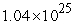 to
to 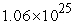
C) 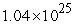 to
to 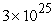
D) 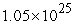 to
to 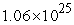
Correct Answer:

Verified
Correct Answer:
Verified
Q85: Use Lagrange multipliers to find the closest
Q86: Use implicit differentiation to find <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5869/.jpg"
Q87: Locate all critical points and classify them.
Q88: Show that the indicated limit exists. <img
Q89: Find an equation for the tangent plane
Q91: Match the surface to its contour plot.
Q92: Match the surface to its density plot.
Q93: Wind chill is a combination of temperature
Q94: Use the contour plot to estimate <img
Q95: Sketch the indicated traces of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5869/.jpg"