Essay
Use Bohr's model to draw a sodium (Na) atom and a chlorine (Cl) atom. Using your model, explain what happens when sodium reacts with chlorine to form table salt. Include in your explanation ion and ionic bond formation. Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 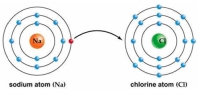
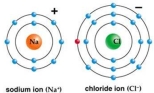
Correct Answer:

Verified
Sodium donates an electron to chlorine t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: Both <sup>18</sup>O and <sup>16</sup>O are found in
Q43: Which of the following elements would be
Q48: What does this graph reveal about the
Q49: This system of chemicals, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4749/.jpg" alt="This
Q50: If the atomic number of an element
Q51: Which statement is NOT true about polar
Q52: Draw two hydrogen atoms using Bohr's model.
Q54: Which substances are on the basic side
Q56: The scale indicates the relative concentrations of
Q57: As a solid, water floats. This means