Multiple Choice
Which of the following sets of quantum numbers is NOT allowed?
A) n = 1,  = 1,m
= 1,m  = 0,ms = -
= 0,ms = - 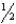
B) n = 3,  = 0,m
= 0,m  = 0,ms = +
= 0,ms = + 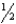
C) n = 3,  = 1,m
= 1,m  = -1,ms = -
= -1,ms = - 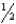
D) n = 5,  = 4,m
= 4,m  = 0,ms = +
= 0,ms = + 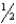
E) n = 6,  = 3,m
= 3,m  = 2,ms = -
= 2,ms = - 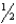
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: What is the longest wavelength of light
Q43: The Fraunhofer lines are evidence that _.<br>A)atoms
Q67: Suppose a blue light emitting photons
Q69: What are the principal and angular momentum
Q70: Electron affinity is the energy change that
Q71: Which of the following statements regarding degenerate
Q74: A hydrogen atom is excited to n<sub>initial</sub>
Q75: You synthesize an atom that appears to
Q76: What is the de Broglie wavelength
Q145: Which statement regarding atomic emission spectra and