Multiple Choice
Which of the following statements regarding degenerate orbitals is correct?
A) Orbitals sharing the same principal and angular momentum quantum numbers are degenerate.
B) Orbitals sharing the same magnetic quantum number are degenerate.
C) Electrons with the same value of n are equal in energy in all atoms.
D) Orbitals sharing the same 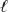 value are degenerate.
value are degenerate.
E) As the effective nuclear charge increases,all orbitals in a shell become equal in energy.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: The Fraunhofer lines are evidence that _.<br>A)atoms
Q66: Calculate the de Broglie wavelength of
Q67: Suppose a blue light emitting photons
Q69: What are the principal and angular momentum
Q70: Electron affinity is the energy change that
Q72: Which of the following sets of quantum
Q74: A hydrogen atom is excited to n<sub>initial</sub>
Q75: You synthesize an atom that appears to
Q76: What is the de Broglie wavelength
Q145: Which statement regarding atomic emission spectra and