Multiple Choice
Use the molecules below to answer the next three questions. 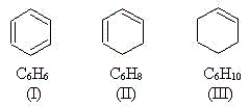
-Which molecule(s) have at least one carbon atom that is sp hybridized?
A) I
B) II
C) III
D) all of the above
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: Consider the molecule and the following hybridization
Q92: Complete the Lewis structure for the following
Q93: Which of the following statements about the
Q94: Which of the following molecules or ions
Q95: Atoms that are sp<sup>2</sup> hybridized form _
Q96: The hybridization of the B in BH<sub>3</sub>
Q97: The electron configuration of a particular
Q98: In the molecular orbital description of CO:<br>A)The
Q99: If four orbitals on one atom overlap
Q101: Consider the structure of glycine,the simplest