Multiple Choice
The following two reactions are important in the blast furnace production of iron metal from iron ore (Fe2O3) : 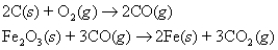 Using these balanced reactions,how many moles of O2 are required for the production of 3.19 kg of Fe?
Using these balanced reactions,how many moles of O2 are required for the production of 3.19 kg of Fe?
A) 42.8 moles
B) 19.0 moles
C) 171 moles
D) 57.1 moles
E) 2.39 moles
Correct Answer:

Verified
Correct Answer:
Verified
Q47: The molecular formula always represents the total
Q48: For the reaction P<sub>4</sub>O<sub>10</sub>(s)+ 6H<sub>2</sub>O(l) <span
Q49: A substance contains 35.0 g nitrogen,5.05 g
Q50: Given the equation 3A + B
Q51: Vitamin C contains the elements C,H,and O.It
Q53: The refining of aluminum from bauxite
Q54: Iron is produced from its ore by
Q55: The _ in a balanced equation represent
Q56: Reaction of methane with oxygen really proceeds
Q57: Consider a specific chemical reaction represented