Multiple Choice
Iron is produced from its ore by the reactions: 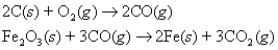 How many moles of O2(g) are needed to produce 4.6 moles of Fe(s) ?
How many moles of O2(g) are needed to produce 4.6 moles of Fe(s) ?
A) 2.3 mole O2
B) 3.5 mole O2
C) 4.6 mole O2
D) 6.9 mole O2
E) 13.8 mole O2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: A substance contains 35.0 g nitrogen,5.05 g
Q50: Given the equation 3A + B
Q51: Vitamin C contains the elements C,H,and O.It
Q52: The following two reactions are important in
Q53: The refining of aluminum from bauxite
Q55: The _ in a balanced equation represent
Q56: Reaction of methane with oxygen really proceeds
Q57: Consider a specific chemical reaction represented
Q58: When 20.0 g C<sub>2</sub>H<sub>6</sub> and 60.0 g
Q59: A 5.95-g sample of AgNO<sub>3</sub> is reacted