Multiple Choice
A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation 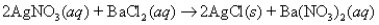 to give 3.17 g of AgCl.What is the percent yield of AgCl?
to give 3.17 g of AgCl.What is the percent yield of AgCl?
A) 45.0%
B) 53.3%
C) 31.6%
D) 63.1%
E) 100%
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Iron is produced from its ore by
Q55: The _ in a balanced equation represent
Q56: Reaction of methane with oxygen really proceeds
Q57: Consider a specific chemical reaction represented
Q58: When 20.0 g C<sub>2</sub>H<sub>6</sub> and 60.0 g
Q60: Chlorous acid,HClO<sub>2</sub>,contains what percent hydrogen by mass?<br>A)1.92%<br>B)25.0%<br>C)23.4%<br>D)1.47%<br>E)5.18%
Q61: NaHCO<sub>3</sub> is the active ingredient in
Q62: Phosphoric acid can be prepared by
Q63: Roundup,an herbicide manufactured by Monsanto,has the formula
Q64: How many of the following statements are