Multiple Choice
The solubility of silver phosphate,Ag3PO4,at 25°C is 1.55 10-5 mol/L.Determine the concentration of the Ag+ ion in a saturated solution.
A) 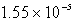 M
M
B) 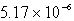 M
M
C) 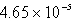 M
M
D) 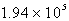 M
M
E) 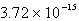 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: The solubility of an unknown salt,M<sub>2</sub>Z,in a
Q34: Calculate the molar concentration of uncomplexed
Q35: Silver acetate,AgC<sub>2</sub>H<sub>3</sub>O<sub>2,</sub> is a sparingly soluble
Q36: Which of the following solid salts should
Q37: When a mixture containing cations of Analytical
Q39: How many moles of CaF<sub>2</sub> will
Q40: The following questions refer to the
Q41: In a solution prepared by adding
Q42: Which of the following solid salts is
Q43: An unknown salt,M<sub>3</sub>Z,has a K<sub>sp</sub> of <img