Multiple Choice
The solubility of an unknown salt,M2Z,in a 0.0768 M CaZ solution is 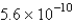 mol/L.Calculate the Ksp for M2Z.
mol/L.Calculate the Ksp for M2Z.
A) 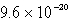
B) 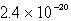
C) 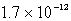
D) 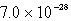
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: The solubility in mol/L of Ag<sub>2</sub>CrO<sub>4</sub>
Q29: The K<sub>f</sub> for the complex ion
Q30: In the classic scheme for qualitative analysis,the
Q31: Which of the following solid salts is
Q32: The cation M<sup>2+</sup> reacts with NH<sub>3</sub>
Q34: Calculate the molar concentration of uncomplexed
Q35: Silver acetate,AgC<sub>2</sub>H<sub>3</sub>O<sub>2,</sub> is a sparingly soluble
Q36: Which of the following solid salts should
Q37: When a mixture containing cations of Analytical
Q38: The solubility of silver phosphate,Ag<sub>3</sub>PO<sub>4</sub>,at 25°C