Multiple Choice
Solubility Products (Ksp) 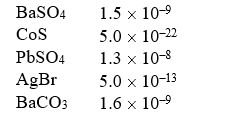
Which of the following compounds is the most soluble (in moles/liter) ?
A) BaSO4
B) CoS
C) PbSO4
D) AgBr
E) BaCO3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: An unknown salt,M<sub>3</sub>Z,has a K<sub>sp</sub> of <img
Q44: Consider a solution made by mixing
Q45: You have a solution consisting of
Q46: Calculate the concentration of Al<sup>3+</sup> in
Q47: The correct mathematical expression for finding the
Q49: The K<sub>sp</sub> of PbSO<sub>4</sub> is 1.3
Q50: Sodium chloride is added slowly to
Q51: The molar solubility of BaCO<sub>3</sub> (K<sub>sp</sub>
Q52: The solubility of an unknown salt,M<sub>3</sub>Z<sub>2,</sub> at
Q53: The solubility of AgCl in water is