Multiple Choice
The Ksp of PbSO4 is 1.3 10-8.Calculate the solubility (in mol/L) of PbSO4 in a 0.0037 M solution of Na2SO4.
A) 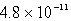 M
M
B) 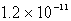 M
M
C) 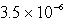 M
M
D) 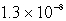 M
M
E) 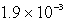 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Consider a solution made by mixing
Q45: You have a solution consisting of
Q46: Calculate the concentration of Al<sup>3+</sup> in
Q47: The correct mathematical expression for finding the
Q48: Solubility Products (K<sub>sp</sub>) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Solubility Products
Q50: Sodium chloride is added slowly to
Q51: The molar solubility of BaCO<sub>3</sub> (K<sub>sp</sub>
Q52: The solubility of an unknown salt,M<sub>3</sub>Z<sub>2,</sub> at
Q53: The solubility of AgCl in water is
Q54: The solubility of Cd(OH)<sub>2</sub> in water