Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Multiple Choice
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
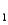 = 4.10 (Ka
= 4.10 (Ka
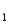 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
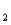 = 11.79 (Ka
= 11.79 (Ka
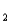 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 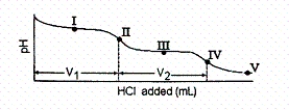
-What is the pH at point III?
A) 4.10
B) 7.95
C) 11.79
D) 12.39
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q1: A 2.36-g sample of an acid,H<sub>2</sub>X,requires 45.0
Q2: What will happen if a small amount
Q3: A 50.0-mL sample of 0.10 M
Q4: Which of the following is true for
Q6: A solution containing 10.mmol of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q7: You have two buffered solutions.Buffered solution 1
Q8: A 50.00-mL sample of 0.100 M KOH
Q9: How many of the following will raise
Q10: A 100.0-mL sample of 0.503 M
Q11: A 100.0-mL sample of 0.2 M