Multiple Choice
A solution containing 10.mmol of 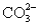 and 5.0 mmol of
and 5.0 mmol of 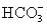 is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
A) 8.8 mL
B) 5.9 mL
C) 2.9 mL
D) 14.7 mL
E) 19.7 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A 2.36-g sample of an acid,H<sub>2</sub>X,requires 45.0
Q2: What will happen if a small amount
Q3: A 50.0-mL sample of 0.10 M
Q4: Which of the following is true for
Q5: Consider the following information about the
Q7: You have two buffered solutions.Buffered solution 1
Q8: A 50.00-mL sample of 0.100 M KOH
Q9: How many of the following will raise
Q10: A 100.0-mL sample of 0.503 M
Q11: A 100.0-mL sample of 0.2 M