Essay
A mouse model of spontaneous rheumatoid arthritis (RA) was fortuitously discovered when a T-cell receptor transgenic mouse line made in C57BL/6 mice (KRN) was crossed to the NOD strain. These mice, known as KxN, developed RA beginning at about 4-5 weeks of age. The disease symptoms included joint swelling, infiltration of inflammatory cells, with eventual deterioration of bone and cartilage. As a first experiment to determine the mechanism leading to this autoimmune response, KRN+ CD4 T cells were isolated from C57BL/6 mice and were stimulated in vitro with purified APCs from wild-type C57BL/6 mice, NOD mice, or C57BL/6 mice expressing the NOD MHC class II allele, I-Ag7 (C57BL/6-Ag7). After three days, the T cultures were pulsed with 3H-thymidine, and cpm incorporated were measured to assess T cell proliferation, as shown in Figure Q3)31)A. Furthermore, the CD4 T cell compartment in KxN mice was examined, and compared to KRN-transgenic negative littermate control mice. Whereas the littermate controls generally had 16-22% CD4 T cells in their spleens, KxN had 5-7% CD4 T cells, all of which expressed the KRN T-cell receptor. 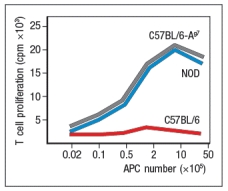
a) What do these data indicate about the mechanism(s) of self-tolerance acting on KRN T-cell receptor-positive CD4 T cells in the KxN mice?
receptor recognized a peptide of a ubiquitous self-antigen (glucose-6-phosphate isomerase; GPI) bound to the NOD MHC class II allele, I-Ag7. In addition, the sera of the KxN mice were found to have circulating IgG antibodies to GPI. To address whether T cells or B cells were required for autoimmune disease initiation and/or progression, a series of studies were performed. In one study, CD4 T cells were eliminated in young KxN mice by injection of a cell-depleting anti-CD4 antibody (or an isotype control antibody), and disease progression was monitored. In a second study, anti-CD4 antibody was injected into KxN mice after disease symptoms had already appeared. The mice were then monitored for the ensuing several weeks. In a third study, KxN mice were crossed to a line of mice with a mutation in the immunoglobulin locus that blocks all B cell development. KxN B-cell-deficient mice were then compared to KxN B-cell-sufficient mice for the development of RA symptoms. These data are shown in Figure. 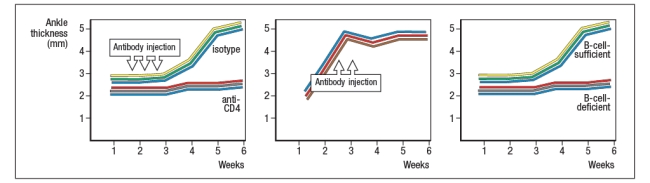
b) What do these data indicate about the role of T cells, B cells, and antibodies in the development and disease progression of RA in this mouse model?
In a next experiment, sera from KxN arthritic mice or non-arthritic control mice were isolated, and injected into RAG-deficient recipients. Recipient mice were then monitored for clinical disease, and were scored on a scale of 1-5 every 3 days, as shown in Figure ; 1 = mildest disease to 5 = severe disease. 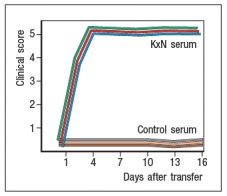
c) What do these data, together with the data shown above, indicate about the pathogenic mechanisms operating in this model of RA?
In a final set of studies, potential effector mechanisms resulting in joint inflammation and destruction were examined. Serum from KxN arthritic mice was injected into Rag-deficient recipients that were lacking expression of the complement component C3 or the neutrophil chemokine receptor CXCR2. Disease progression was monitored after serum injection and compared to that seen following KxN serum injection into control Rag-deficient recipients as shown in . 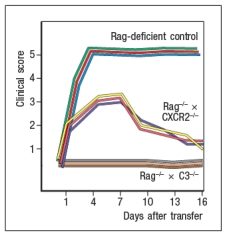
d) What do these data indicate about the disease mechanism in this model of RA?
e) Given all of these data, what is the likely role of the KRN+ CD4 T cells in this disease model?
Correct Answer:

Verified
a) There is evidence for partial clonal ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q20: Based on these data, Fc
Q21: In some cases, a transient autoimmune process
Q22: Polymorphisms in the IL-2R <span class="ql-formula"
Q23: Some early studies aimed at deciphering
Q24: Systemic lupus erythematosis (SLE) is an autoimmune
Q26: A mouse model for multiple sclerosis
Q27: Rheumatoid arthritis is often classified as
Q28: 10) A mouse model for autoimmune hemolytic
Q29: A mouse model for type 1
Q30: Genetic linkage studies have identified numerous genes