Multiple Choice
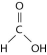 Figure 2.5
Figure 2.5
-Figure 2.5 shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Which of the following takes place as
Q56: The atomic number of sulfur is 16.
Q57: About 25 of the 92 natural elements
Q58: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6147/.jpg" alt=" Figure 2.8 -In
Q59: Which of the following correctly describes chemical
Q60: One of the buffers that contributes to
Q64: A covalent chemical bond is one in
Q80: Van der Waals interactions result when<br>A) hybrid
Q124: Sulfur is in the same column of
Q130: How many electron pairs are shared between